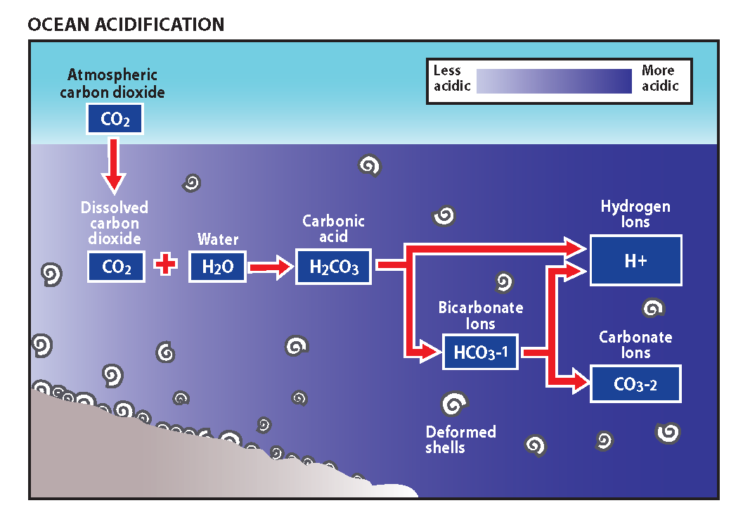
Gases from earth’s atmosphere are absorbed in ocean waters. The amount of carbon dioxide in the earth’s atmosphere has increased substantially since the industrial age that began roughly 150 years ago. Dubbed the “evil twin” of global climate change, ocean acidification results from carbon dioxide added to earth’s atmosphere being absorbed by ocean waters. Roughly a third of the carbon dioxide added to earth’s atmosphere from human causes has been absorbed by ocean waters. The capacity of the ocean to absorb and be a “sink” for atmospheric carbon dioxide will decrease in the 21st century. Although ocean acidification and climate change are often lumped together, they are by no means the same thing. This ocean “sink” has slowed the accumulation of carbon dioxide in our atmosphere and its effects on earth’s climate, but the result is a change in the chemical balance of seawater that is unique to the ocean environment. More information about the potential effects of both global climate change and ocean acidification on Oregon’s nearshore species and habitats can be found in the 2012 supplements to the Oregon Nearshore Strategy (see Appendices A-D).
Carbon dioxide dissolved in seawater is a component of an equilibrium chemical reaction. The balance shifts to create more carbonic acid as the amount of dissolved carbon dioxide increases. More acidic seawater decreases the availability of the carbonate ion building blocks that are necessary for marine organisms to form their skeletons and shells (see diagram). Deep ocean waters naturally have lower carbonite ion availability and are more acidic. Spring and summer upwelling that brings deep nutrient rich waters to Oregon’s nearshore waters also brings more corrosive acidic waters. Exposure to more acidic water has been shown to inhibit shell formation, reduce individual size and population abundance, and to cause behavioral changes that affect survival in marine organisms. California mussels, gooseneck barnacles, pelagic marine snails called pteropods that are food for salmon, hermit crabs and marine fishes are among the organisms for which these effects have been documented. A new study conducted at NOAA‘s Northwest Fishery Science Center found that ocean acidification may slow development and reduce survival of Dungeness crab larvae.
The video Ocean Acidification – Changing Waters on the Oregon Coast provides information on the causes of ocean acidification, its effects on marine life in our coastal waters and why Oregon is at the forefront of these changes taking place in our oceans.
The major findings and recommendations of the West Coast Ocean Acidification and Hypoxia Science Panel, released in April 2016, provide additional information and steps that can be taken to address this issue.